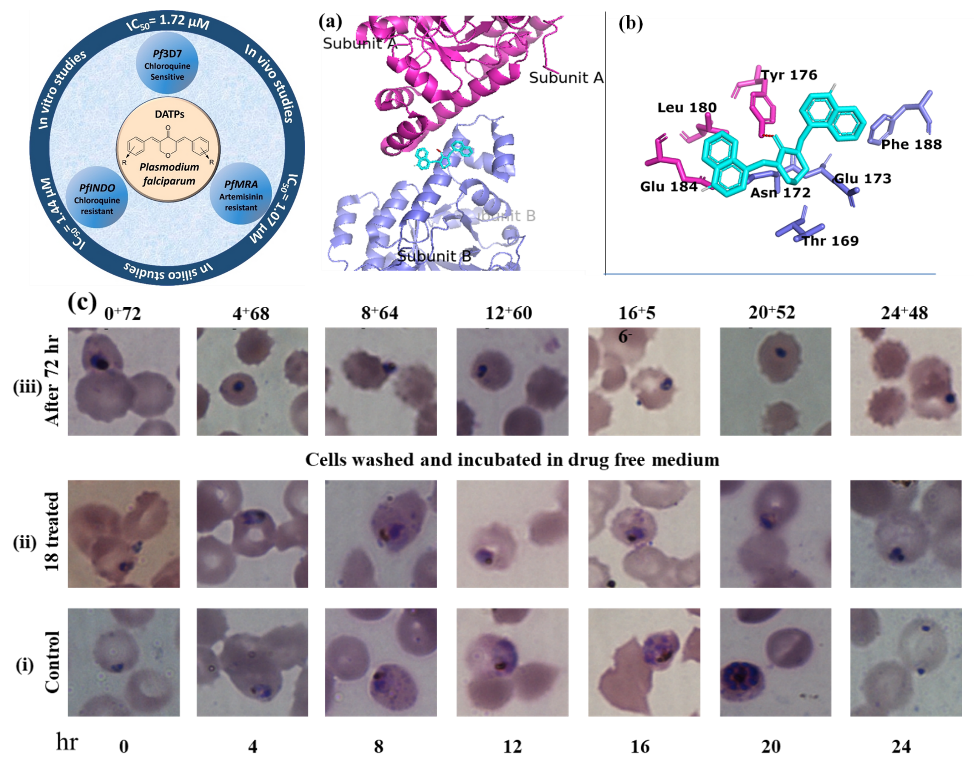
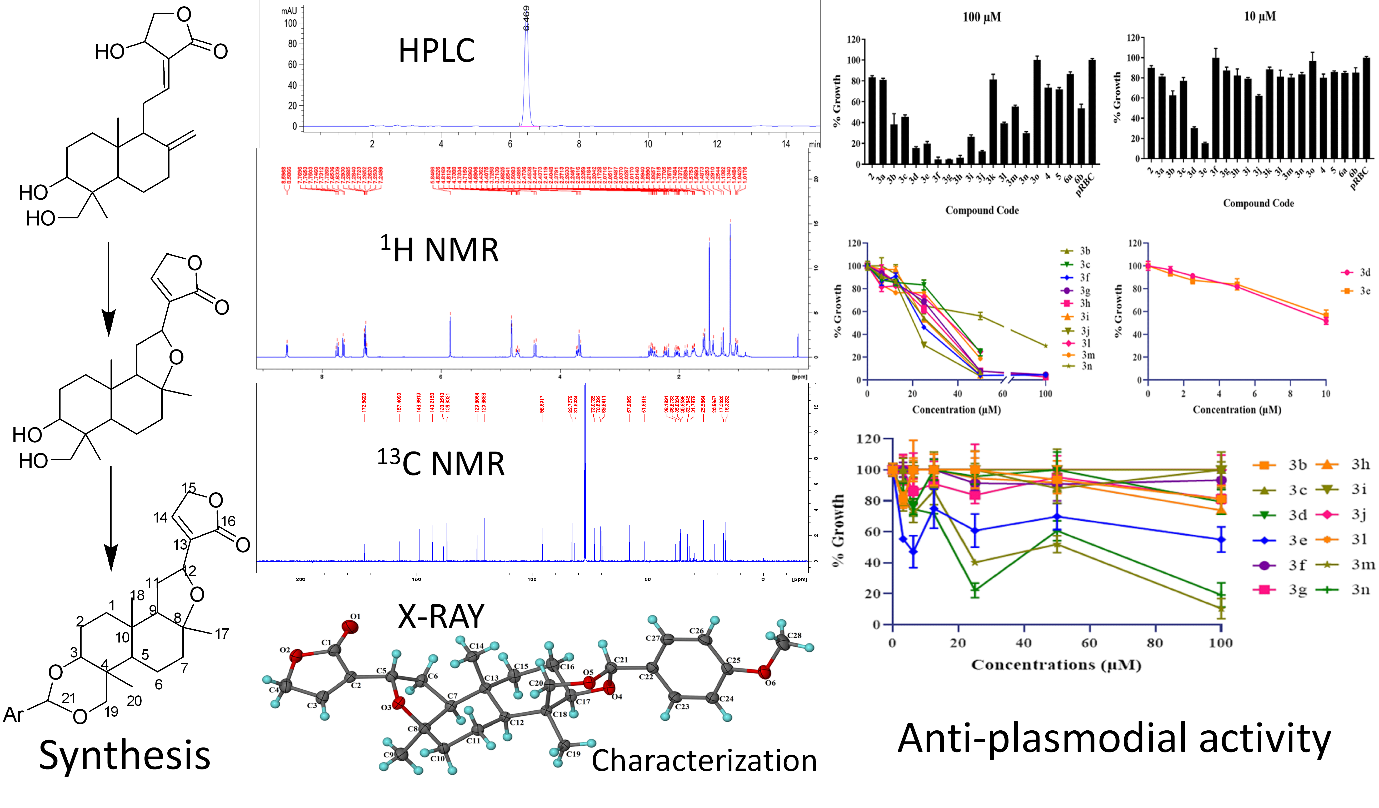
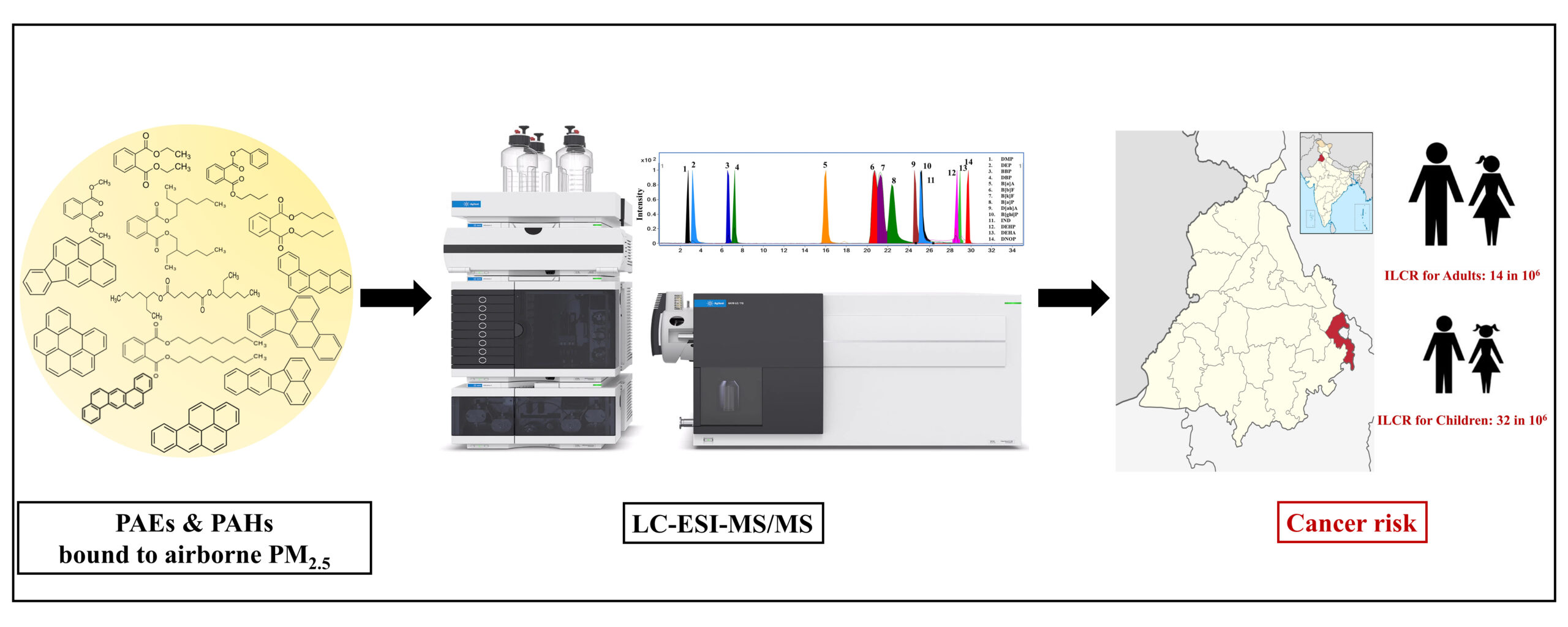
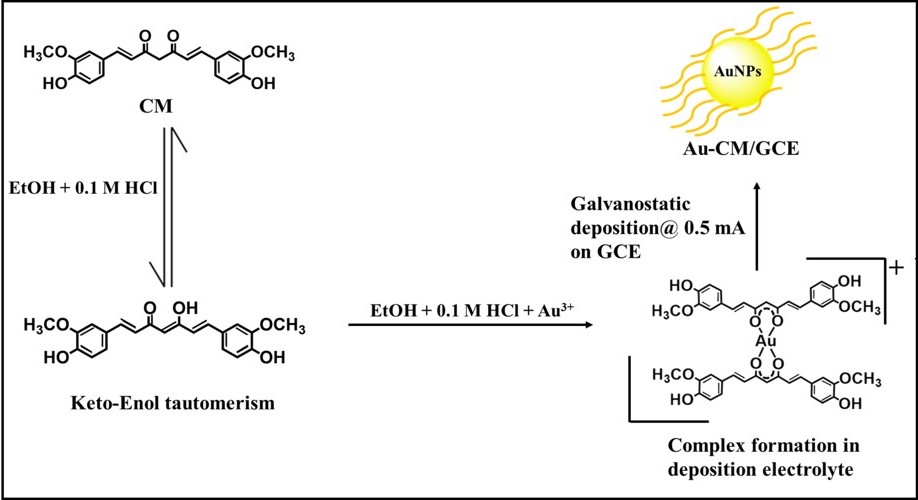
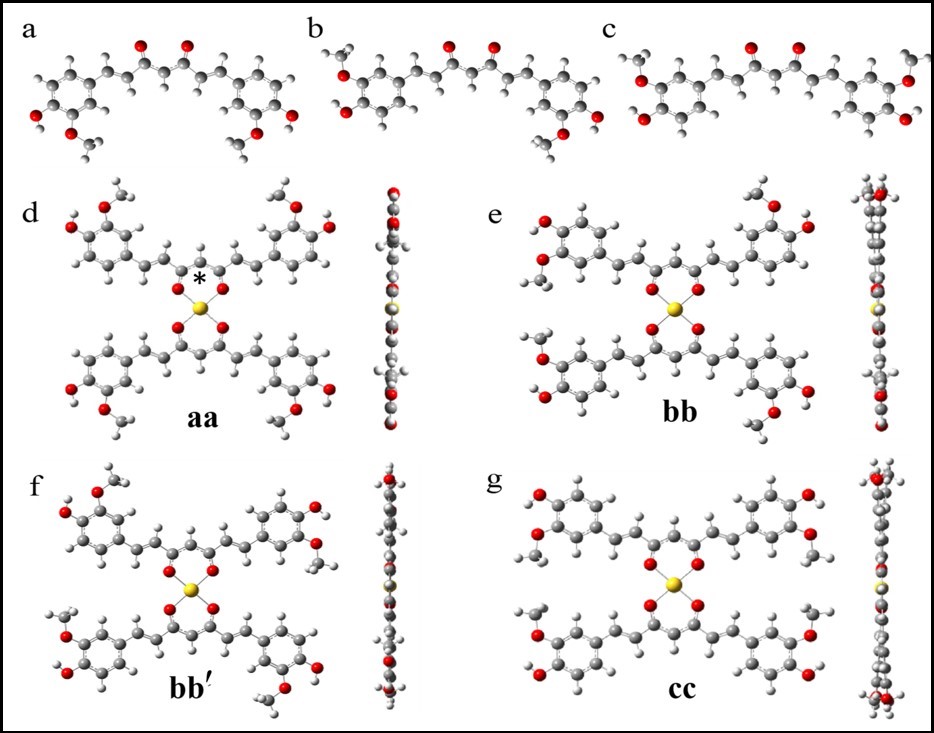
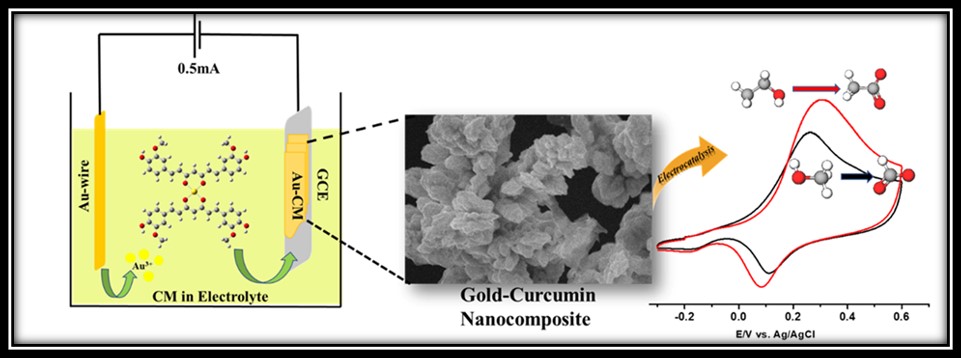
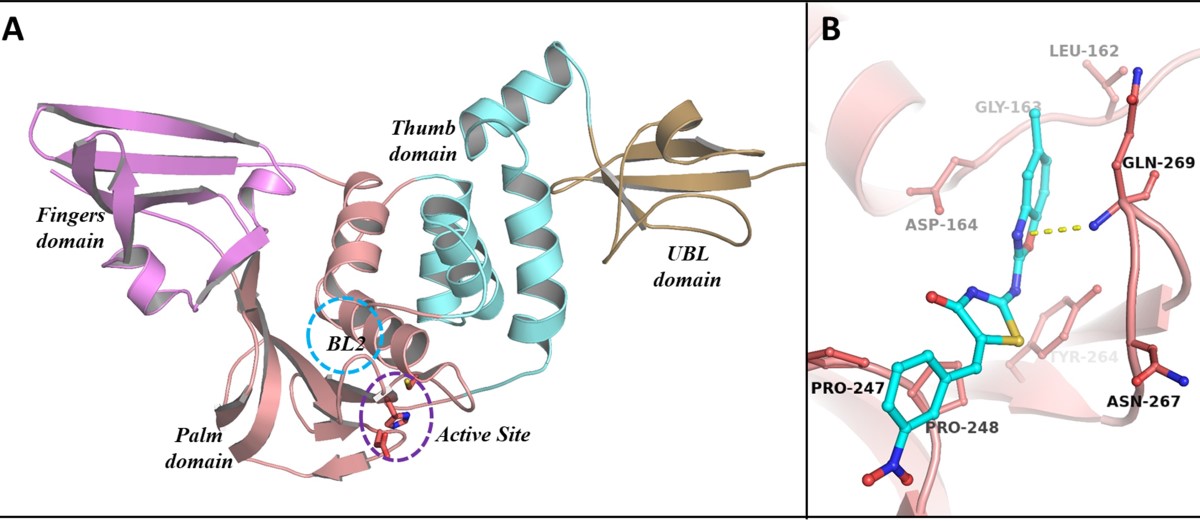
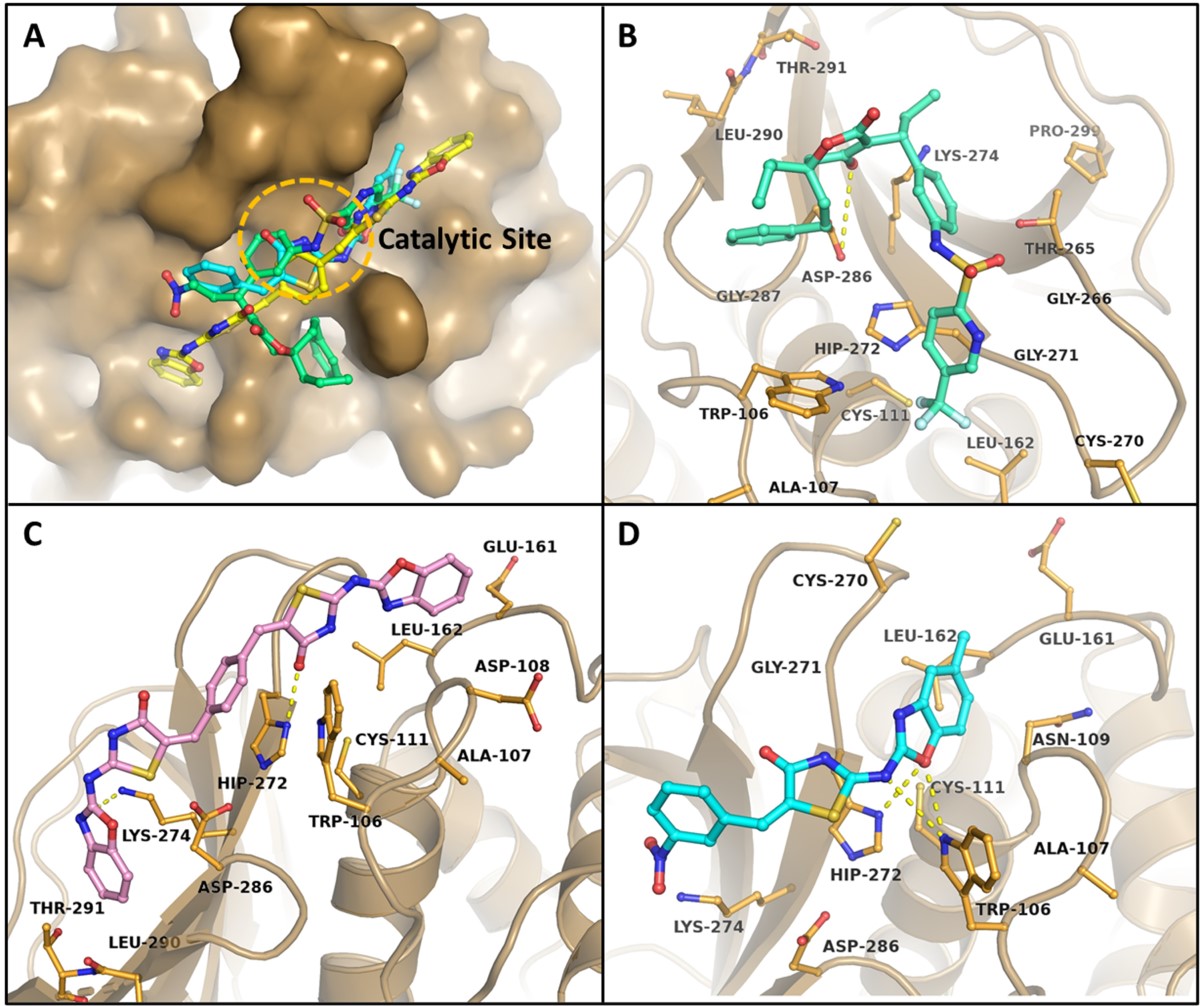
Identifying the Issue
- Spread of multi-drug resistance in the malaria parasite
- Lack of novel therapies and drugs
Objective of the Research
- Development of a novel drug-like candidate with rapid kill kinetics against the malaria parasite Plasmodium falciparum.
- Non-haemolytic and non-toxic to animals.
Who should read this?
Research community working in the Antiplasmodial drug development, medicinal chemists, and pharmaceutical industry.